What Is Relative Atomic Mass
Relative Atomic Mass Properties of Matter Chemistry FuseSchool 124079 views Aug 10 2014 Learn the basics about Relative Atomic Mass. The relative atomic mass of an element is the average mass of all atoms of that element compared to a standard unit.

El Calculation Of Relative Atomic Mass From Mass Spectrometry Data Youtube
Sometimes abbreviated RAM or ram also known by the deprecated synonym atomic weight is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant.
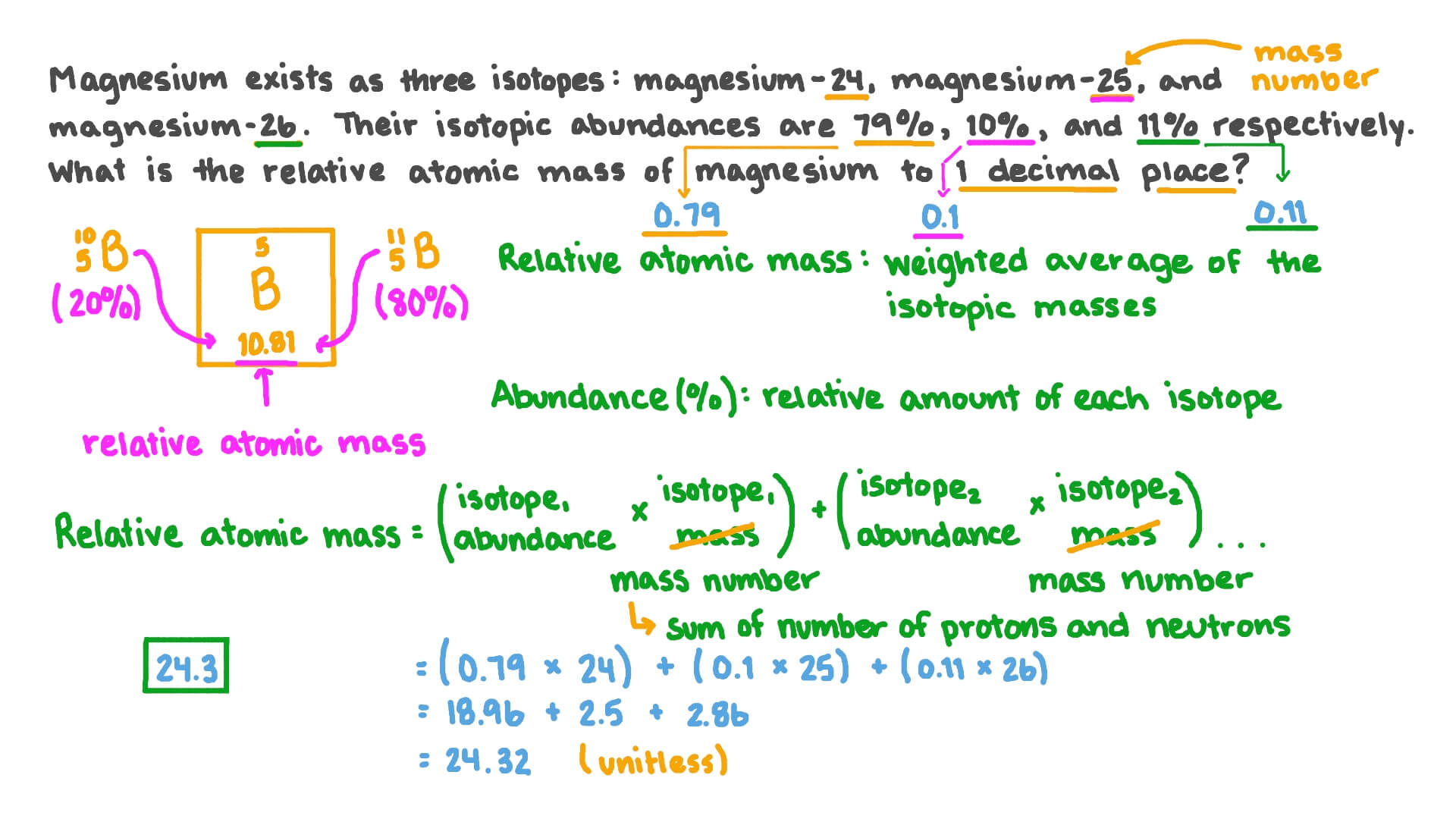
. Relative atomic mass Ar is the weighted average of the masses of the isotopes of an element compared to 112 of the mass of the carbon-12 atom. The relative abundance of an isotope is either given or can be read off the mass. The mass of an atom can be accounted for by the sum of the mass of protons and neutrons which is almost equal to the atomic mass.
The mass numbers of its isotopes the abundance of these isotopes Chlorine Chlorine naturally exists as two isotopes _. In todays video we will discuss the concept of relative atomic mass atomic mass unit how to find the mass of an atom standard unit a. A relative atomic mass also called atomic weight.
The relative atomic mass of an element is a weighted average of the masses of the atoms of the isotopes because if there is much more of one isotope then that will influence the average. This definition of relative isotopic mass is a completely different from the definition of relative atomic mass except both are based on the same international standard of atomic mass ie. The relative atomic mass of an element can be calculated by using the relative abundance values.
Since relative atomic mass is a ratio so it is. The weighted average of the masses of an elements isotopes in comparison to the mass of a carbon-12 atom is known as relative atomic mass RAM or A r The ratio of the average. The standard unit for relative atomic mass is the carbon-12.
Thus relative atomic mass of an. Since both quantities in the ratio are masses the resulting value is dimensionless. Based on the carbon-12 scale the relative atomic mass A r of an element is defined as the average mass of one atom of the element when compared with one twelfth of.
Why is the word relative used for mass. Ar is a measure of how heavy atoms are. Relative atomic mass accounts for the mass and relative abundance of different isotopes of an element.
Relative atomic mass symbol. What is relative atomic Mass. It is the ratio of the average mass per atom of an element from a given sample to.
This small change is due to the binding energy. The relative atomic mass of carbon is 12 while the relative atomic mass of. The relative atomic mass of an element is the ratio between the average mass of its isotopes to 112th part of the mass of a carbon - 12 atom.
Based on the carbon-12 scale the relative atomic mass A r of an element is defined as the average mass of one atom of the element when compared with one twelfth of. The formula for relative. The relative atomic mass Ar of an element is calculated from.
The relative atomic mass of an element shows its mass compared with the mass of atoms of other elements. Mu is defined as being 112 of the mass of a carbon-12 atom. The reason for atomic mass not being a whole number is because atomic mass is reported as a weighted average of all the isotopes.
The relative atomic mass of an element is defined as the average relative mass of an atom of the element compared with an atom of 126 C taken as 12 u. The atomic mass constant symbol. It is a tricky concept thats actually quite.
For example suppose 75 percent of the.

1 Average Atomic Mass Chemistry Notes 2 Relative Atomic Mass Masses Of Atoms Expressed In Grams Are Very Small For Example One Atom Of Oxygen Ppt Download
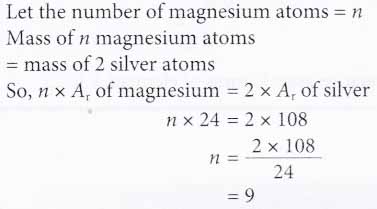
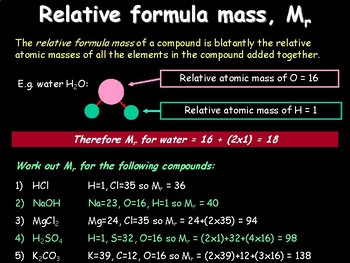
What Is The Relative Atomic Mass And Relative Molecular Mass Of An Element A Plus Topper
Question Video Calculating The Relative Atomic Mass Of Magnesium From Isotopic Abundances Nagwa

2 1 Calculating Relative Atomic Mass Sl Youtube
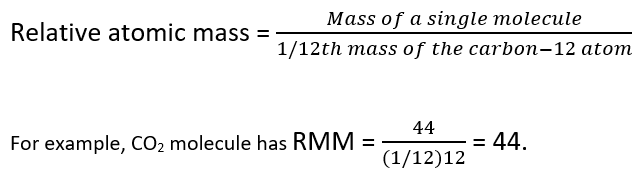
Relative Atomic Mass Relative Molecular Mass Mass Spectrometry A Level Chemistry Revision Notes
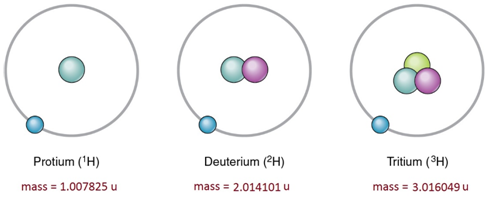
Difference Between Relative Atomic Mass And Atomic Mass Definition Calculation Example

Relative Atomic Mass

Pin On Teaching Ideas

Mole Concept And Chemical Calculations Difference Between Relative Atomic Mass Relative Molecular Mass Relative Formula Mass And Molar Mass

Question Video Calculating The Relative Atomic Mass Of Chlorine From Isotopic Abundances Nagwa

A Level Chemistry Revision Relative Atomic Mass Youtube
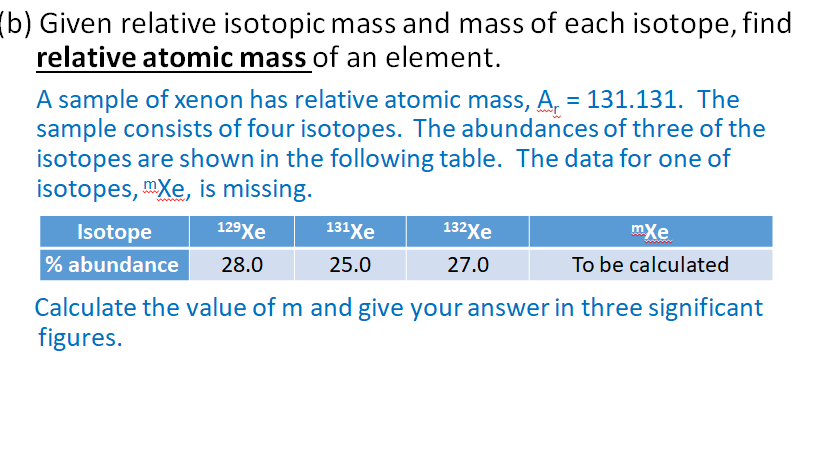
Solved B Given Relative Isotopic Mass And Mass Of Each Chegg Com

Solution Relative Atomic And Formula Mass 1 Studypool

Definition Of Isotopes And Relative Atomic Mass Solutions
Relative Atomic Mass Teaching Resources
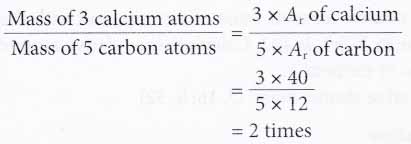
What Is The Relative Atomic Mass And Relative Molecular Mass Of An Element A Plus Topper

1 2 1 Define The Terms Relative Atomic Mass A R And Relative Molecular Mass M R Youtube